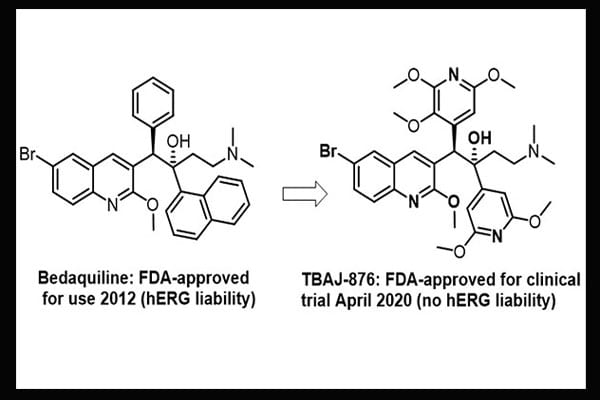
On 29 April 2020 the US Food and Drug Administration granted the drug TBAJ-876 approval to proceed to clinical trial for tuberculosis. TBAJ-876 was developed in the Auckland Cancer Society Research Centre by Drs Sutherland, Tong, Choi, Blaser, Conole, Tsang, Denny and Palmer, in collaboration with the Global Alliance for Tuberculosis Drug Development. It is an improved analogue of the tuberculosis drug bedaquiline (Janssen).
Bedaquiline is a very selective inhibitor of the ATP synthase enzyme of Mycobacterium tuberculosis. It has revolutionised the treatment of drug-resistant tuberculosis around the world but has a cardiotoxicity liability due to its inhibition of the hERG potassium channel. TBAJ-876 has superior anti-tubercular activity to bedaquiline both in vitro and in vivo and is also much less cardiotoxic, with greatly attenuated hERG blockade. Phase I human trials of TBAJ-876 are expected to begin in June 2020.
Read the paper